Michael Reed, Fred Nijhout, Cornelia Ulrich
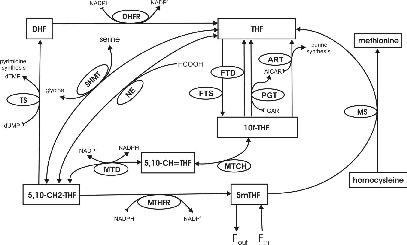
The methionine cycle is important for the regulation of homocysteine, an important risk factor for heart disease, and for the control of DNA methylation. Both hyper- and hypomethylation have been proposed as crucial steps in chains of events that turn normal cells into cancerous cells. The purpose of the project is to use mathematics to understand normal folate and methionine metabolism, DNA methylation, and purine and pyrimidine synthesis and then to understand how they are affected by alterations in diet and gene abnormalities.
Anita Layton and Danny Lew
Many of the molecular players in this pathway are known, but the remarkable sensitivity of the process in the face of large amounts of noise is not understood. We are combining a mathematical model of the Turing process that establishes polarity in yeast with a stochastic model of vesicle delivery and retrieval to generate and test hypotheses about the process in silico. In parallel, we use genetic manipulation and sophisticated imaging of living cells undergoing chemotropism to test our ideas in vivo. Recent technical advances have allowed us to probe relevant protein dynamics with unprecedented resolution, revealing unexpected oscillatory aspects of polarization, which we would also like to model.
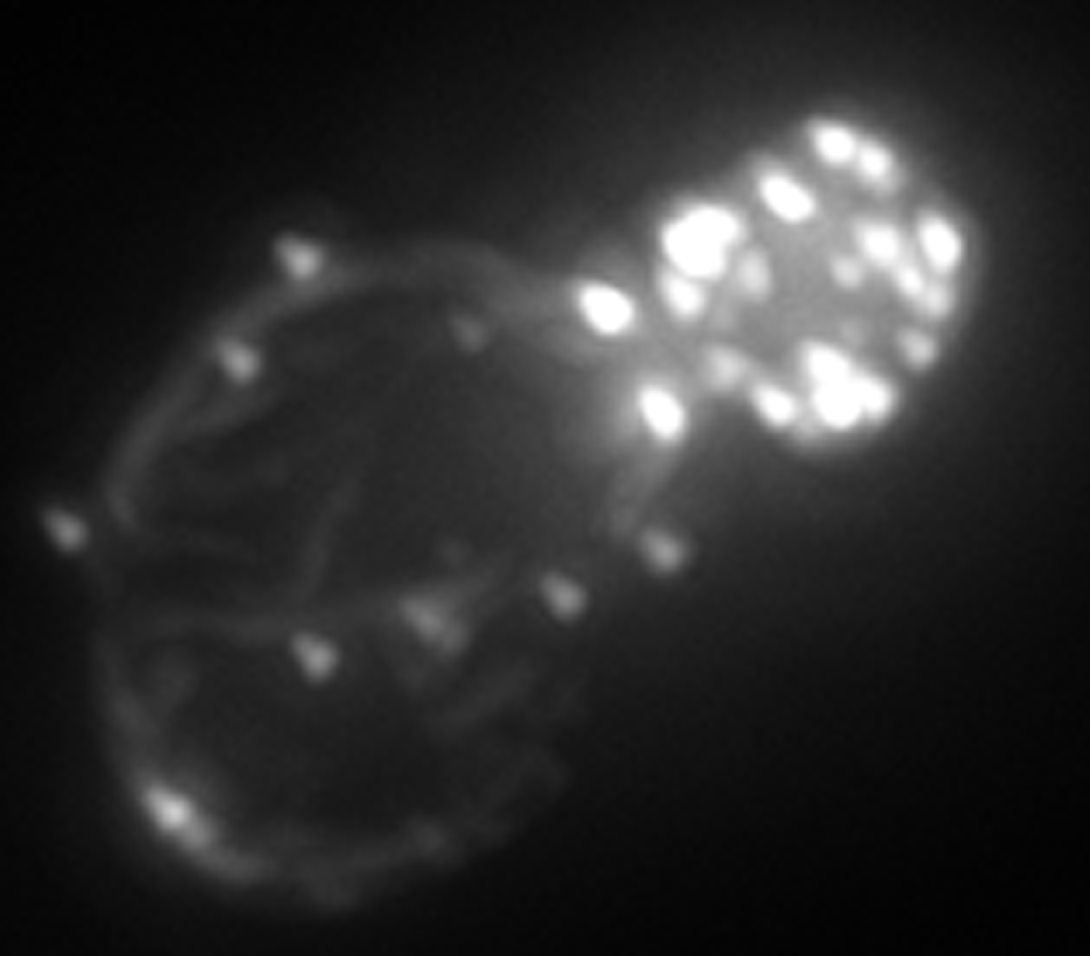
Stephanos Venakides, John Harer, Anita Layton, Glenn Edwards, and Dan Kiehart
John Harer leads the effort in the development of topology-based segmentation methods for data sets and their application to the study of dorsal closure. Information from the segmentation analysis provides a framework for structuring and testing of mathematical models. Harer is extending previous segmentation methods by including global structure using persistent homology, a method that improves data quality based on global, rather than local, considerations. The goals of the dorsal closure segmentation project are to: (a) Accurately identify cell boundaries that have been tagged with fluorescent proteins. (b) Assemble z-slices and 3D segmentation of the epithelial cells in the embryo, (c) Develop appropriate descriptors of cell-shape, and use them to understand the evolution of cellular shape and interactions through time.