Harold Layton and Anita Layton
Dynamic models for the concentrating mechanism involve large systems of coupled hyperbolic PDEs that describe tubular convection and epithelial transport. Numerical solutions of these PDEs help to integrate and interpret quantities determined by physiologists in many separate experiments.
Anita Layton and Harold Layton
This project aims to provide a more complete and quantitative understanding of the means by which the kidney of a mammal can produce urine that is more concentrated than blood plasma (i.e., that contains more solute per unit volume than does blood plasma). While the hypothesis of the countercurrent multiplication for the outer medulla is believed to be well established, the urine concentrating mechanism in the inner medulla, where the largest axial osmolality gradient is found, remains controversial. We seek to better understand this long-standing mystery by working with experimentalists.
This basic research is relevant to public health, because abnormalities of the kidney's urine concentrating capability are known to cause, contribute to, be a consequence of, or occur along with, a number of important disorders and diseases, including abnormal body water and salt retention or loss.
David Schaeffer and Daniel Gauthier
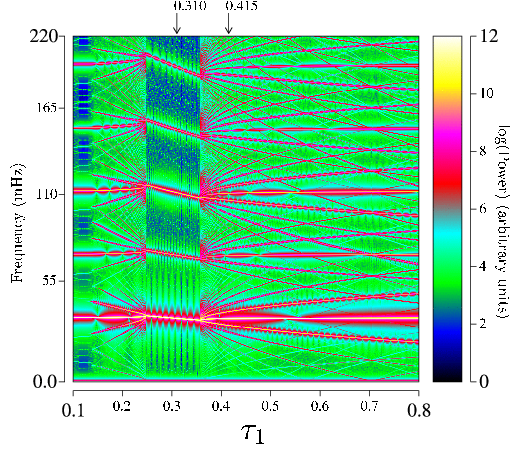
One intriguing application of the chaos control methods we have developed is in the biological area. We have initiated a program to characterize in vitro the dynamics of small pieces of rapidly paced cardiac muscle and to use feedback methods to suppress or control the observed bifurcations by applying small perturbations to the tissue. We find that there are only a small number of classes of bifurcations in the tissue, but that there is significant variation in the prevalence of these behaviors from animal to animal.
In addition, we are using similar methods to control in vivo a fibrillating sheep atrium. The eventual long term goal of this project is to develop an implantable defibrillator that will maintain a healthy rhythm in humans prone to the onset of atrial fibrillation using only small electrical shocks. In our current experiments with sheep, we use a high-density mapping system to record the spatial-temporal complexity occurring on the surface of the heart during atrial fibrillation. Small control shocks are applied to a single electrode attached to the surface of the heart based on real-time measurements of the cardiac dynamics at a nearby spatial location.
Mauro Maggioni, Fred Nijhout, and Michael Reed
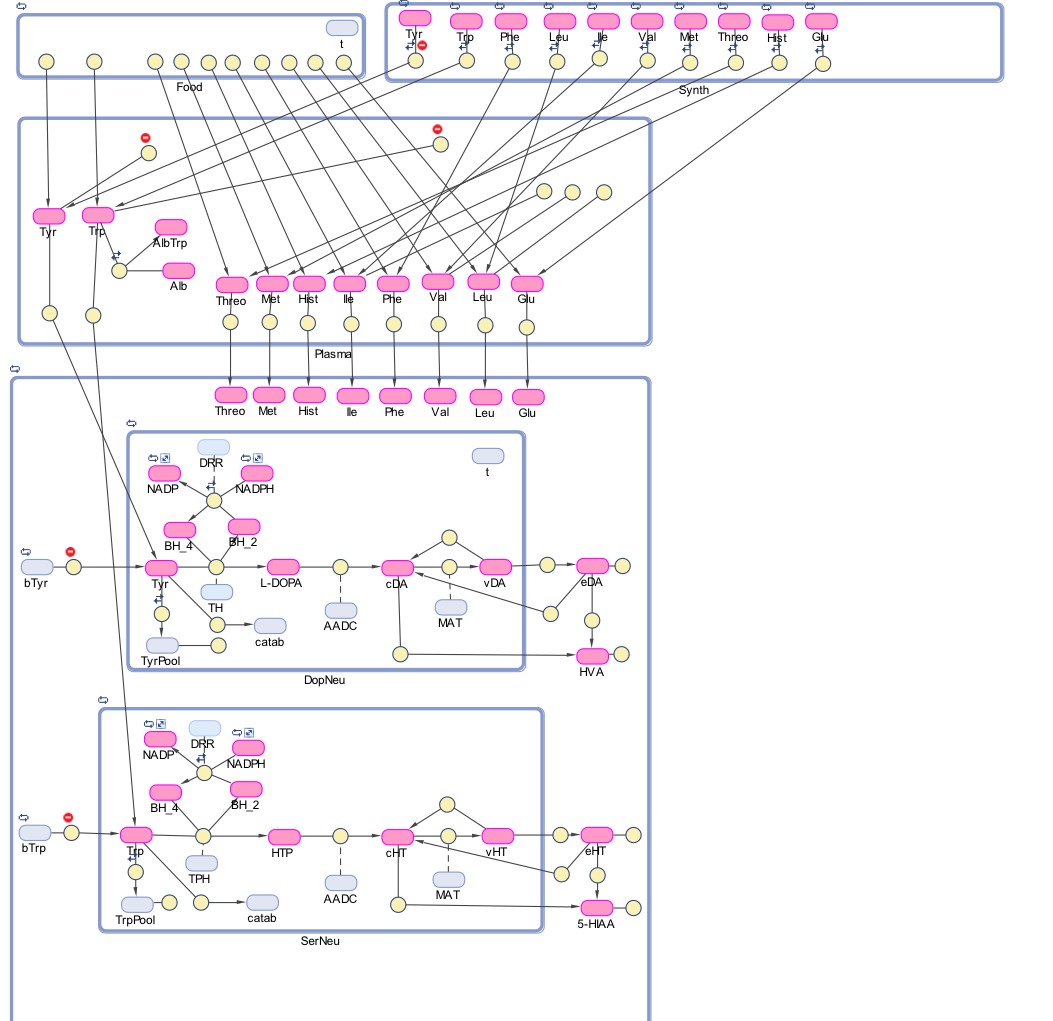
The Blood Brain Barrier insulated the nervous system through very specific membranes that regulate the transport of chemicals from the blood to the brain (and vice-versa). We are interested in modeling the transport of aminoacids, especially of those (such as tyrosine and tryptophan) that are precursors of important neurotrasmitters (dopamine, serotonine, norepinephrine...). Moreover we are interested in modeling how food intake regulates blood concentrations of such aminoacids, and in turn how that affects uptake in the brain.
The blood brain barrier has a competitive system which controls the uptake into the brain of a family of amino acids (the large neutral amino acids) as well as tyrosine and tryptophan. Competition implies that large relative quantities of some amino acids will decrease the uptake of amino acids with relatively smaller concentration (in fact, the kinetic parameters for the transport of each amino acid are of course important, not just the concentration), and therefore diets with large amounts of certain amino acids (e.g. branched chain amino acids) may lower, in the short and long term, the uptake of important precursors of neurotrasmitters in the brain.